DISPOSABLE HOSPITAL EQUIPMENTS
STERILE GLOVES, NEEDLE, NEEDLE COVER, CATHETER(PTFE), FLASHBACK CHAMBER, WINGS, INJECTION PORT, SYRINGES, THREADED STOPPER, SCALP VEIN SET, LIVER LOCK, INFUSION SET WITHOUT AIR VENT, VOMIT BAG, ORAL SNAB, OXYGEN MASK, MUCUS EXTRACTOR, ORAL ENDOTRACHEAL TUBE, SURGICAL BLADES, SCALPEL HANDLES, ABSORBENT COTTON BALLS, PARAFFIN DRESSING GAUZE, STERILE EYE PADS, NON VOVEN ADULT DIAPERS, CAP AND MASK, SURGICAL GOWNS
For medical devices, single use disposable device offer additional and essential advantage. Risk of infection and sterilization requirements often bake the use of recycled products in medical care impossible. Disposable products are therefore used frequently in medical care and offer safe medical treatment. However, medical materials are also subject to environment requirements and ambitions to improve their environmental impact.
I.V. Set is a medical Disposable precisely used for glucose and other liquid inject- able to the body. The main problem associated with such devices is to prevent motion, irritation, infection, and the formation of a sinus tract with weeping of fluid. These objectives can be achieved by anchoring the devices in various ways. For example, the attachment of material containing holes or fenestrations for tissue in growth or a textured surfaces interest with the surrounding tissues and a bond is formed. Unfortunately, however, these texturised surfaces may be extruded gradually as a natural consequence of cell maturation which results in cells migrating toward the surface.
A central venous catheter is a plastic tube placed in a large, central vein in the neck, chest, or groin. Most catheters are tunneled—they go under the skin of the chest and into a vein in the neck. The part used for HD comes out through the skin of the chest. It has two pieces of tubing in a Y-shape with a cap on each end.
A catheter can be held in place by a couple of stitches if it will be used for a very short time. In most cases, the catheter will have a cuff around the tubing that goes under your skin. Your tissue will grow around the cuff and hold it in place. Once a catheter is in, only a doctor should take it out.
As per international standard ASTM F 2100–07, surgical face masks are generally classified in to 3 types. They are i) Low barrier, ii) Moderate barrier and iii) High barrier. The basic characteristics to distinguish the surgical face masks based on its barrier properties are listed in Table 2 (ASTM F 2100, 2007). Quality evaluation: European standards and ASTM standards provides the standardize quality evaluation procedure for surgical face masks to prevent transmission diseases from health care professionals to patients and in certain situations vice-versa. Also provide the critical requirements before marketing the surgical face masks (EN 14683, 2005). There are five test methods used to evaluate the performance of the surgical face masks.
For medical devices, single-use disposable devices offer additional and essential advantages. Risk of infection and sterilization requirements often make the use of recycled products in medical care impossible. Disposable products are therefore used very frequently in medical care and offer safe medical treatment. However, medical materials are also subject to environmental requirements and ambitions to improve their environmental impact. Medical devices have many different requirements to fulfill, such as medical treatment performance and technical functionality, handling and operational performance, medical/patient safety, environmental impact and cost efficiency. All of these aspects have to be weighed against each other and optimized.
Astra Tech is a large producer of medical devices with a world-wide market. The environmental aspect is of major concern for Astra Tech and an important factor in the development of new products. The aim of the present research work was to establish a base for the internal choice of material in the production and to show the environmental performance for the different alternatives. However, ecological aspects are complex and require advanced handling methods. In order to be able to analyze the present product situation and to design new products with high environmental performance, it is important to establish a method for evaluating the environmental impact. For this purpose, IVL Swedish Environmental Research Institute carried out the present study.
In this study, we analyzed the environmental performance of three different plastic materials for urinary catheters. These three plastic materials represent a bulk plastic material (polyvinylchloride, PVC), a high quality performance plastic (thermoplastic polyurethane, TPU) and a newly developed plastic material based on the experiences from the present environmental evaluations (a polyolefin-based elastomer). In this article we show how the material selection for a product and the development of a new, environmentally improved, material can be based on LCA results.
The Medical Devices and Equipment industry, valued at US$ 2.5 billion contributes only 6% of India’s US$ 40 billion healthcare sector. Moreover, it is growing at a faster annual rate of 15% than 10-12% growth seen in the Healthcare sector in its entirety. A rise in the number of hospitals and the increased requirement for healthcare facilities creates a need for sophisticated devices and equipment, which can provide accurate treatment to individuals. The Medical Electronics segment of this industry incorporates control, conversion, sensing, processing, storage, display, and transfer of information on anatomy and physiology by making use of the Electronics and Communication Technologies. The Medical Equipment industry is quite wide with > 14,000 different products types, as per the Global Medical Device Nomenclature (GMDN). The products range from wound closure pads to stents and IVD machines of medical devices. Further, it can be reasonably said that Medical Electronics is an area, where Electronics and Information Communication Technology play a decisive role.
The market for disposable medical gloves will be worth $4 billion by 2017, according to projections from a report published by Global Industry Analysts (GIA).
POTENTIAL CONSUMERS
Hospitals are the major consumers of surgical gloves, but they are also used by:
– Medical & chemical laboratories.
– Medical clinics.
– Chemical industry.
– Food industry
– Households for protection purposes.
GLOBAL MARKET OF MEDICAL DISPOSABLE
The global medical disposables market deals with the market estimation for disposable medical products used in medical procedures. The market is majorly driven by the shift of patient and practitioner focus from the use of reusable medical products to disposable products. This report provides market size estimation for medical disposables in terms of revenue (USD billion), for the period 2012 – 2018, considering 2011 as the base year.
The market is segmented on the basis of product types into drug delivery products, wound management disposables, non-woven medical disposables and others. The market for these product segments is provided for four geographic regions, namely, Europe, North America, Asia-Pacific and Rest of the World (RoW). The market size and forecasts for all these segments has been provided in USD billion along with the % CAGR for the forecast period 2012 to 2018.
MANUFACTURING PROCESS OF DISPOSABLE, HOSPITAL EQUIPMENTS (NEEDLE COVERS, FLASH BACK CHAMBER WINGS, THREADER STOPPER LIVER LOCK SCALPEL HANDLE & INJECTION PORT.)
For the manufacture of disposable hospital equipments, a requisite amount of plastic polymer are fed into injection moulding machine to get the requisite materials depending upon the moulds used in the process.
Finally, the product is ejected from the moulds and finishing operations is accomplished by scrap grinder.
There is a huge demand of all the health care medical plastic products/equipments in India and abroad both. Engineers India Research Institute, Delhi (India) have recently prepared Market survey cum Detailed techno Economic Feasibility Report on DISPOSABLE HOSPITAL EQUIPMENTS (STERILE GLOVES, NEEDLE, NEEDLE COVER, CATHETER(PTFE), FLASHBACK CHAMBER, WINGS, INJECTION PORT, SYRINGES, THREADED STOPPER, SCALP VEIN SET, LIVER LOCK, INFUSION SET WITHOUT AIR VENT, VOMIT BAG, ORAL SNAB, OXYGEN MASK, MUCUS EXTRACTOR, ORAL ENDOTRACHEAL TUBE, SURGICAL BLADES, SCALPEL HANDLES, ABSORBENT COTTON BALLS, PARAFFIN DRESSING GAUZE, STERILE EYE PADS, NON VOVEN ADULT DIAPERS, CAP AND MASK, SURGICAL GOWNS) based on the following capacity:
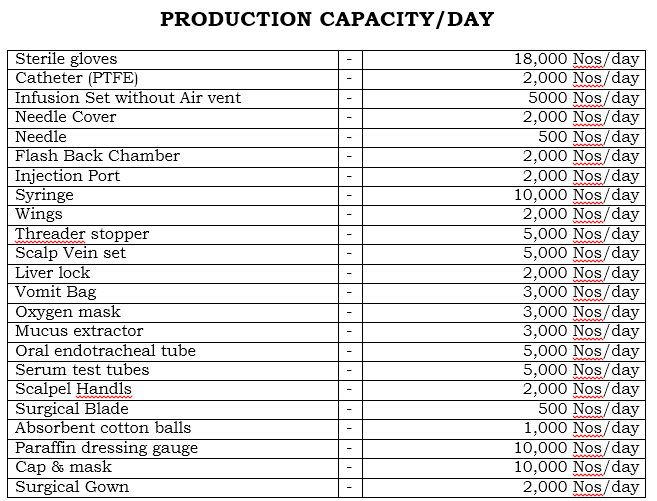
Where Land area is 6000 Sq. mtr required, Plant and Equipments would be US$ 9.17 Lacs, Working capital per Month US $ 2.33 lacs, Total capital Investment with Land, Building, Machinery and 2 Month Working Capital is US $ 22 Lacs, Rate of Return 65% and Break Even Point is 35%.
To Obtain the copy of this Industrial Detailed Techno Economic Feasibility Report, May call on +91 9811437895 Sudhir Gupta